All about gas detection
Find out everything you need to know about gas detectors and how they work.
Why do we need gas measuring instruments at all? An Introduction.
In the course of evolution, the human body has adapted itself to the earth’s atmosphere. The human organism works best in roughly 21% oxygen and 79% nitrogen. In this context ubiquitously occurring trace gases such as CO2 and noble gases are neglected. Small deviations in the composition of our breathing air may impair the well-being, larger ones will damage health and very large changes in the atmosphere can be deadly.
Toxic gases can be extremely harmful to health – even in very low concentrations. Safety specialists have identified the following potential hazards:
- Lack of oxygen
- Presence of explosive gases
- Presence of toxic gases
The above mentioned deviations may occur in nature as well man – made. In todays industrial society anthropogenic pollution is predominant. To start with, here some typical examples of natural atmosphere changes:
- In rotting processes are produced combustible gases (methane) as well as toxic gases (hydrogen sulphide, carbon dioxide, mercaptans, etc.). Mainly CO2 and H2S gas tend to accumulate in pits or sinks. A typical accident is the so-called cesspool accident: someone comes too close to a cesspool, inhales hydrogen sulfide which impedes respiration. The victim falls into the cesspit and drowns or suffocates. Persons who try to help him or her, suffer the same fate. Nonetheless it is not usual to carry gas detectors on farms.
- The situation is quite different in sewage plants. In their clearing basins digestion processes similar to a cesspool take place. Even the rake area close to the intake must be monitored for flammable gases. In this area mostly stationary gas detectors are installed. The incoming wastewater might carry fuels or solvents or have even started to rot and release methane. In the working areas the employees carry multi gas detectors to be warned from gases which may be released by the sewageprocess. Typically these instruments measure combustible gases, oxygen deficiency, carbon dioxide and hydrogen sulfide.
1. An excursion into history
Even in ancient Greece was known that alcoholic fermentation may produce dangerous gases. In these times it was not known what a gas is, but people had a vague feeling of danger. And they were right! So already ancient time winegrowers placed a candle on the floor of their wine cellars. When it went off, due to a lack of oxygen, they had to leave the cellar. Of course, in these times they could not know that the danger was caused by carbon dioxide. Just as little did they know that carbon dioxide is toxic from 0.5%, but that the candle flame dies only at about 10%.
Another typical example of naturally occurring gases is mining. Therefore it is no surprise that the mining industry made very early attempts to recognize the danger of gas as soon as possible. Already a short time after the birth of Christ, people knew about the health hazard caused by mercury in mines. This is described in a writing of Pliny the Elder about 100 A.D. Even later authors (1556 Georgius Agricola: “De Re Metallica”, around 1700 and Bernardino Ramazzini: “De Morbis Artificium Diatribe”) knew about the dangers of some dangerous substances in mining. They called it fire damp. Today we know that it consists of toxic carbon monoxide and explosive methane.
However, primarily they had no idea what a gas was. They just knew there was something threatening around. Consequently they did not know what to look for. They helped themselves by taking small animals underground. They had noticed, that the toxic or suffocative atmosphere was more dangerous the smaller an organism was. So they took small animals underground, canaries for instance. When the animal showed signs of weakness, it was high time to leave the mine.
But when later the industrial revolution created more and more demand for coal, more and more mines popped up, and more and more people were working underground. The need for an accurate measurement of dangerous gases increased. Even before it was known that gas caused the danger, they noticed, that a dangerous substance changed the appearance of a candle flame as soon as it was present. By the middle of the 18th century it was noticed that this dangerous substance changing the appearance of the candle flame could be used to detect this substance – whatever it was.
Specialists, so called “firemen”, could determine whether the atmosphere was dangerous or not, by watching the the shape and color of the flame. If there was gas, they tried to burn it, before they let miners enter the area. This method was everything but accurate and safe, and carrying a candle in a potentially not only combustible but explosive atmosphere could have fatal consequences. To be a fireman was a very dangerous profession.

In order to avoid the candle to be a source of ignition, they tried to separate the flame from the atmosphere by columns of water. This construction was very complicated to operate. In the end, Humphrey Davy’s idea of separating the flame from the atmosphere by an arrangement of close-meshed metal nets prevailed. In 1816 the first Davy lamps were tested in England. For more than 150 years the Davy lamp in its original form and with some improvements over time, was to be the only way to detect gas.
There were several attempts to make it easier to read the height of the flame by adding a scale. The installation of a bimetal strip could already be called a precursor of flame temperature measurement. (The more methane was present, the more fuel was available, the hotter the flame burned, the stronger was the excursion of the bimetal strip).
Now miners had a tool for lighting which also worked as a device that could warn from the presence of dangerous gas. With some experience the concentration of gases could be estimated. If the flame just burned higher, explosive Methane was present, if the flame color turned bluish, there was toxic (and also combustible) Carbon Monoxide. However, this method depended on the ambient air containing enough oxygen. Unfortunately this is by no means guaranteed in mines. Fortunately, since then the methods of gas measurement technology have become more and more delicate. So let`s go back to present time.
2. How do modern gas detectors work?
2.1 Measurement of combustible gases
2.1.1 Catalytic sensors
These sensors take advantage of the property of combustible materials to release energy, when they are burned. Therefore they are also called “heat of oxidation” sensors. During the combustion of e.g. methane, the following happens:
CH4 + 2 O2 -> CO2 + 2 H2O + Energy
The more methane is in the air, the more energy is released during this reaction. The next step was creating an arrangement which could measure the released energy exactly. Two ceramic beads (also called pellistors) are used for this purpose – both are heated to approx. 400°C by an internal platinum coil. One of the coils is coated with a catalyst. On its surface, the methane is oxidized, i.e. energy is released – a process that further heats the bead. This heating increases the electrical resistance of the internal coil. The second bead is not catalyst – coated. Therefore it cannot oxidize the gas. This way, the signal of the catalytic sensor can be compensated for external temperature changes, air flow, humidity, etc., because the impact of these parameters is the same on both beads.
To make sure the beads cannot be a source of ignition, they are separated from the atmosphere by flame barriers.
In this context the actual concentration of the gas is unimportant. You want to know if there is a danger of explosion or not. Therefore the measuring range is set to 0 – 100 % LEL (= lower explosion limit). Unfortunately, the LEL is a substance-specific parameter. So you have to know which gas you are dealing with. Then you have to look up in the literature at which concentration the LEL of this gas is. (A very comprehensive source for the properties of hazardous substances is e.g. the GESTIS file of the professional associations.
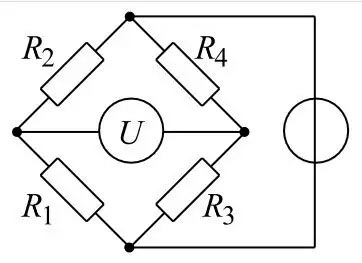
LEL detection becomes difficult when there are gas mixtures. Since the composition of the atmosphere is unpredictable, a so-called safety calibration will solve the problem. In this case you do not adjust your measuring instrument to the gases expected in the mixture. You select a substance that is detected with less sensitivity. Nonane is often used, because it has a very low LEL. However, since Nonane is a liquid, calibration to Nonane in the field would be very cumbersome. It is difficult and therefore highly inaccurate to produce an accurate gas mixture from a liquid in the field. Therefore Compur Monitors offers a “double calibration”. In the laboratory, the calibration is performed with Nonane and a second time with a reference gas, e.g. Butane. The reference factor is noted on the sensor so that the user can calibrate in the field with an easy to use air/butane mixture from a standard bottle.
Catalytic sensors have many advantages:
Of course there are also disadvantages:
2.1.2 Infrared sensors
Many gases absorb light at a specific wavelength. Each molecule takes a certain number of light quanta from a light beam. This energy is used to make certain electrons oscillate. Fortunately, this effect only happens at a substance – specific wavelength. By choosing the right wavelength, you can determine which gas the instrument shall measure. For the measurement of hydrocarbons you choose 3.4 µm – at this wavelength the C – H bonds absorb infrared light.
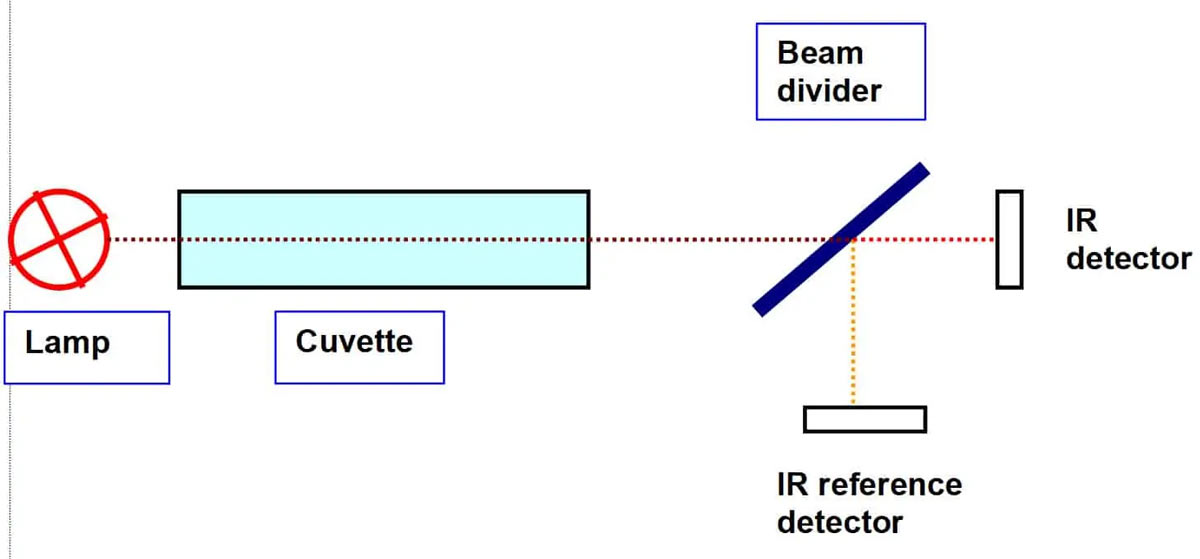
By measuring the amount of absorbed energy, one can “count” the number of gas molecules in the light path: You send a light beam through a cuvette with a light detector at its end. The less light the detector receives, the more gas is in the cuvette. In order to compensate for other environmental influences like humidity, pressure or temperature, a second beam with a different wavelength is used and directed to a second light detector. Light of a certain wavelength can be created by filters, optical gratings, prisms or LED light sources of a certain color.
Alternatively a single light beam can be split behind the cuvette into two beams of different wavelengths.
Infrared sensors have several advantages:
Naturally, there are also disadvantages:
Flame temperature sensors
As already described for the Davy lamp, the presence of a flammable gas increases the temperature of a flame. Usually this is a hydrogen flame. An accurate temperature sensor in the flame can easily detect this increase of temperature
Flame temperature sensors have several advantages:
Naturally there are also disadvantages:
3. Toxic gas sensors
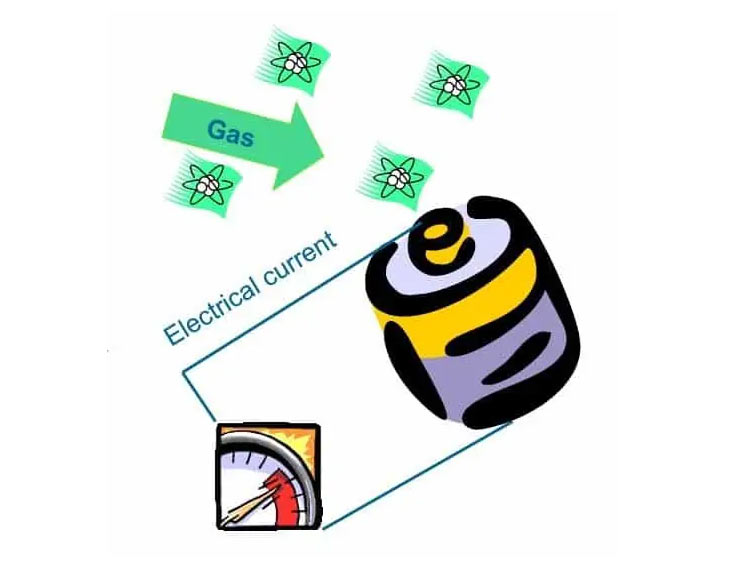
3.1 Electrochemical sensors
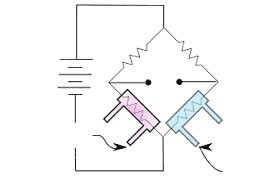
Electrochemical sensors operate as a battery, powered by gas. As long as no gas is present, nothing will happen. As soon as the gas to be measured hits the working electrode, a chemical reaction takes place, which produces or consumes electrons. In below sketch the process at the working electrode is shown schematically.
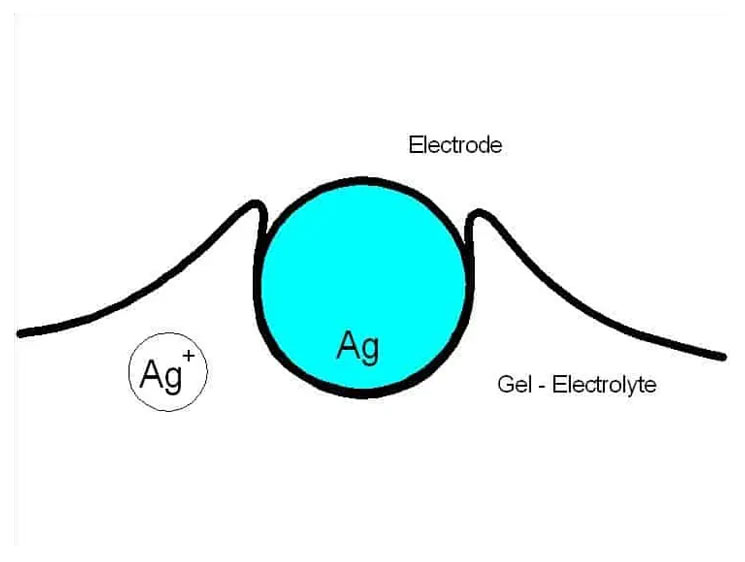
What exactly is happening here?
Everyone knows this from his home: Silver cutlery turns black if stored in air. A chemical reaction takes place, silver oxide is produced. In our sensor the silver oxide is dissolved in the electrolyte, which now contains silver ions: 2 Ag0 -> 2 Ag+ + 4 e– This process will go on until a stable equilibrium of elementary silver and silver ions is reached. Now a gas comes to the party. It reacts with the silver ions:
H2S + 2 Ag+ -> Ag2S + 2 H+
The silver sulfide is non soluble. The ions involved are removed from the equilibrium. In order to maintain the equilibrium the working electrode is forced to produce new ions and electrons. The electrons produced, flow through a very sensitive meter to the counter electrode. The so produced electrical current is not very strong, typically a few nA.
Since each gas molecule generates a certain number of electrons, the current is proportional to the number of gas molecules hitting the electrode. Knowing the chemical reaction allows to predict which gas this sensor can measure: All gases that form a non soluble salt with silver. In fact this sensor technology is used to measure H2S, HCl, HCN and COCl2. Therefore this measuring method is very specific. By choosing the appropriate electrode materials and electrolyte, sensors can be made specific to certain gases. Unfortunately, not all gases are reactive enough to react with the electrode material. Sometimes it helps to add a third electrode and apply a voltage to it, in order to force the chemical reaction. But there are limitations to that method. Therefore, electrochemical sensors can mainly be used to measure inorganic gases and oxygen.
Electrochemical sensors have some advantages:
Of course there are also disadvantages:
3.2 PID Sensors
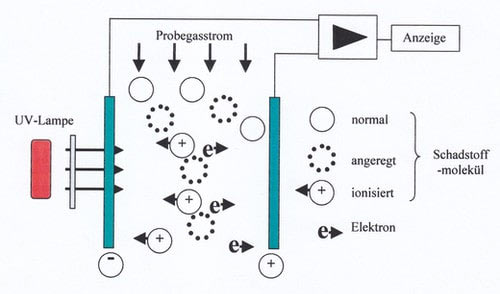
PID sensors use the fact that very high energy UV light can split gas molecules into radicals. These do have an electrical charge. Charged particles can be easily counted by passing them through a cuvette, to the wall of which a high voltage is applied. Positively charged particles will move to the electrode with a negative charge and vice versa, where they discharge. This discharge current is proportional to the number of molecules i. e. the concentration.
Lamps with 10.6 or 11.7 eV can be used as UV sources. This energy is not sufficient to ionize water, oxygen or nitrogen molecules. Therefore the PID does not detect substances which are always present in air. A PID will detect many substances, especially many hydrocarbons. Unfortunately all of them are displayed with different sensitivities.
Most hydrocarbons are liquids. A calibration with a liquid is difficult to perform and should be avoided whenever possible. It was therefore been agreed to calibrate all PID`s with isobutene and to determine the reference factors of as many substances as possible. Isobutene is not toxic, and in low concentrations non – combustible and very stable. Therefore it is the preferred, easy – to handle span gas. All you need is the appropriate reference factor of the measuring gas to Isobutene. These factors can be found in the literature, or e.g. here.
PID Sensors have some advantages:
Naturally there are also disadvantages:
3.3 Thermal Conductivity Sensors
Many gases conduct heat differently compared to air. This effect can be used to determine the concentration when measuring the thermal conductivity of the atmosphere.
In order to do this, a heating element and a thermometer are placed in a cell, through which the gas is pumped. If the sample in the measuring cell contains a gas conducting heat better or less than air, the thermometer in the measuring cell will measure a different temperature.
Thermal conductivity sensors have some advantages:
Of course there are also disadvantages:

Dr. Josef von Stackelberg
Managing Director COMPUR MONITORS GmbH & Co. KG
Contact us without obligation
Do you have any questions about our products or would you like a non-binding consultation? We look forward to hearing from you.